U.S FDA Approves Gilead’s Groundbreaking HIV Prevention Shot, Yeztugo
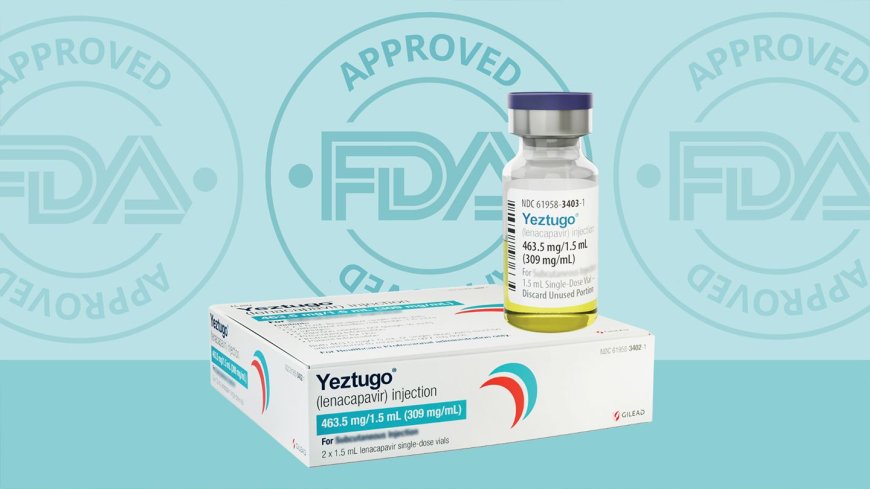
In a major breakthrough in the fight against HIV, the U.S. Food and Drug Administration (FDA) on Wednesday approved Gilead Sciences’ lenacapavir, a twice-yearly injectable drug for the prevention of HIV infection in adults and adolescents. The drug will be marketed under the brand name Yeztugo.
By Mavis Emefa Goka / June 23, 2025
In a major breakthrough in the fight against HIV, the U.S. Food and Drug Administration (FDA) on Wednesday approved Gilead Sciences’ lenacapavir, a twice-yearly injectable drug for the prevention of HIV infection in adults and adolescents. The drug will be marketed under the brand name Yeztugo.
The approval marks a pivotal moment in the global response to the HIV epidemic, offering a new, long-acting prevention option that could significantly reduce transmission rates.
Lenacapavir belongs to a novel class of drugs known as capsid inhibitors and demonstrated near-100% effectiveness in large clinical trials conducted last year.
“This is a milestone moment,” said Daniel O’Day, Chief Executive Officer of Gilead Sciences. “We believe that lenacapavir is the most important tool we have yet to bend the arc of the epidemic and move this epidemic into the history books.”
The drug, which functions as pre-exposure prophylaxis (PrEP), has been widely praised by public health experts and advocates for its potential to improve adherence and accessibility. Unlike daily oral PrEP regimens, Yeztugo requires only two injections per year, a major advancement in convenience and long-term prevention strategy.
The prestigious journal Science named lenacapavir the 2024 Breakthrough of the Year, further highlighting its impact and scientific significance.
HIV/AIDS activists and investors alike had been closely watching the FDA’s decision, with many hopeful that the new treatment could accelerate efforts to end the 44-year-old HIV epidemic, which continues to affect more than 1.3 million people globally each year.
Gilead says it is preparing for a rapid U.S. launch and is working with global health partners to expand access worldwide.
“We are committed to ensuring Yeztugo reaches the people who need it most,” said O’Day. “This approval represents hope—not just for individuals, but for entire communities striving for an HIV-free future.”
With the FDA’s green light, experts anticipate that lenacapavir could reshape the landscape of HIV prevention, especially among high-risk populations who may face challenges with daily medication adherence.
What's Your Reaction?
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0